The Role and Impact of Arginine on Dental Caries Therapeutics
Increasing scientific evidence has indicated that delivering exogenous arginine is a promising approach to prevent caries and even reverse the development of early lesions.
ABSTRACT
As a multifactorial disease, dental caries remains a global public health challenge despite extensive preventive measures, including worldwide use of fluoride toothpaste and mouthrinses, fluoridated water supplies, and application of sealants. Thus, novel and more effective approaches are needed to expand the arsenal to fight the problems associated with dental caries. About 40 years ago, researchers found that alkali generated by arginolytic bacteria through arginine catabolism can raise the pH of the oral biofilm microenvironment by neutralizing glycolytic acids, thus preventing an ecological microbial shift toward the acidogenic bacteria associated with tooth demineralization. Over the past several decades, increasing evidence from in vitro and in vivo studies has indicated that delivering exogenous arginine is a promising approach to prevent caries and even reverse the development of early lesions. The caries-preventive effect of arginine can be attributed to its ability to improve plaque pH stability, inhibit virulence factors of cariogenic pathogens, and modulate the oral microbiota toward a health-promoting microbial profile. For clinical applications, arginine can be incorporated into topical formulations, such as dentifrices, mouthrinses, and varnishes, and it functions much like a prebiotic. Furthermore, development of probiotics that can catabolize arginine and generate alkali is another potential direction for caries prevention. While several arginine-based oral care products are commercially available, their long-term anticaries efficacy needs to be validated in large-scale clinical studies.
Dental caries remains a global public health burden. According to the 2017 Global Burden of Disease report, the prevalence of untreated caries in primary and permanent teeth was 7.8% and 29.4%, respectively.1 Although the caries prevalence rate has largely decreased in the past 50 years due, in large part, to the systemic and topical use of fluoride around the world,2 caries remains one of the most common diseases globally because of inadequate prevention and treatment and an increase in sugar consumption. The key mechanism of fluoride in caries prevention is promoting remineralization on the tooth surfaces while having a minimal effect on the oral cariogenic microbiome. Hence, comprehensive measures targeting multiple caries risk factors are urgently needed to complement the anticaries effect of fluoride.
Novel strategies that have been proposed to further reduce caries prevalence and severity primarily include calcium- and phosphate-based chemistries that increase tooth mineral saturation3 and therapeutics to precisely target the oral microbiome.4-6 With new insights into caries ecological etiology,7 there is a growing appreciation of the crucial role of the eubiotic oral microbiome in maintaining oral health. Novel strategies for caries prevention that focus on microbial modulation rather than indiscriminate killing of oral bacteria are receiving increasing attention. These new strategies for combating dental caries include reengineering the microbiome by targeted suppression of specific cariogenic bacteria using vaccines,8 bacteriophages,4 or targeted antimicrobial peptides.5 Other probiotic approaches include the displacement of Streptococcus mutans with engineered S mutans strains of reduced pathogenesis or with species that either compete with or directly antagonize S mutans.6
Despite the distinct conceptual strengths of each of the aforementioned approaches, they have not been developed into approved therapeutics to prevent dental caries yet. One agent, arginine, has recently received considerable attention for its potential to prevent caries by modulating the microbial community. Compelling in vitro and in vivo data are accumulating and indicate that arginine use leads to a healthy, noncariogenic microbiome by modifying the plaque microflora through promoting the growth of beneficial bacteria rather than having broad-spectrum bacterial killing. Thus, arginine displays great potential to develop into an effective therapeutic tool to complement fluoride applications for preventing dental caries.
The goal of this review is to provide researchers and clinicians with an update of the role and impact of arginine in caries therapeutics. We will elaborate on the mechanisms by which arginine improves homeostasis of the oral microbiome and summarize the applications and clinical outcomes of arginine-based technology for dental care.
(Please note: The articles in this JADA+ series on arginine were presented at a symposium and did not undergo peer review.)
ARGININE AND ITS ROLE IN ORAL HEALTH
As a semi-essential amino acid, arginine (L-arginine) is derived from exogenous dietary protein intake, endogenous body protein breakdown, and de novo arginine biosynthesis from citrulline.9 Host-derived arginine can be secreted into the oral cavity via saliva as a free amino acid. Exogenous and endogenous arginine-containing peptides/proteins can be broken down by proteases produced by oral microbiota to release free arginine. The concentration of free arginine in stimulated, parotid ductal saliva of healthy adults is about 14.61 nM/mL.10
In the late 1970s and 1980s, Kleinberg’s group reported that alkali generated by arginolytic bacteria played a major role in maintaining oral microenvironmental pH, especially in the presence of fermentable carbohydrates in in vitro studies.11-13 In a subsequent clinical study, Van Wuyckhuyse and colleagues10 showed that caries-free adults had significantly higher free arginine levels in stimulated, parotid ductal saliva than individuals with a history of dental decay. The concentration of arginine within dental plaque formed 48 hours after oral hygiene procedures was measured and determined to be approximately 0.22 mM/mg plaque,14 indicating that arginine can be taken up by oral biofilms. Furthermore, the amount of generated ammonia (NH3), a product from arginine catabolism, was higher in plaque samples than in saliva samples from the same individuals.15 Because caries is a biofilm-dependent oral disease, uptake and active metabolism of arginine in dental plaque suggests its potential impact on the plaque microbiome, implying a close relationship between arginine and oral health. These early-stage laboratory and clinical findings opened a new research field in oral health that spans basic, translational, and clinical studies pertaining to the role and impact of arginine in caries therapeutics.
METABOLISM OF ARGININE BY ORAL BACTERIA
Arginine can be metabolized by bacteria through 2 pathways: the arginine deiminase system (ADS) and the agmatine deiminase system (AgDS). Studies reported that ADS activity is higher in the plaque and saliva of caries-free individuals than in caries-active children16 and adults.15 Expression of ADS can be induced by arginine and low pH,17 which plays an important role in pH homeostasis via microbial modulation. AgDS has been associated with acid resistance in bacteria. Its activity in oral biofilms is generally lower than that of ADS.18
The arc operon of ADS is composed of 4 genes: arcA, arcB, arcC, and arcD/arcE. Arginine-ornithine antiporter, a membrane-embedded protein encoded by arcD/arcE, is involved in transporting arginine from the extracellular environment into cytoplasm. Intracellular arginine can be catabolized by arginine deiminase, an enzyme encoded by arcA, into citrulline and ammonia. Citrulline can be further broken down by arcB-encoded ornithine carbamoyl transferase into ornithine and carbamoyl phosphate; the former is then transported out of the cell by arginine-ornithine antiporter through electroneutral ornithine/arginine exchange. Carbamoyl phosphate is further catabolized by arcC-encoded carbamate kinase into ammonia and carbon dioxide (CO2), concomitantly donating the phosphate to adenosine diphosphate and producing adenosine triphosphate19 (Figure 1). Genomic and phenotypic analyses revealed the presence of ADS in multiple bacteria species, most of which are commensal, including Streptococcus sanguinis, Streptococcus gordonii, Streptococcus mitis, Streptococcus oralis, Streptococcus rattus, Streptococcus parasanguinis, and Streptococcus cristatus; certain Lactobacillus and Actinomyces species; and a few spirochetes.7,20
Arginine in dental biofilms also can be catabolized by the bacterial enzyme arginine decarboxylase into agmatine, which can be further broken down via AgDS. The key genes that encode AgDS-related functions, aguA, aguB, aguC, and aguD, are often organized into one operon.17,21 AguD encodes an agmatine-putrescine antiporter, which enables the entry of free agmatine into the cell and transport of putrescine out of the cell. Agmatine can be hydrolyzed to N-carbamoylputrescine and ammonia by agmatine deiminase encoded by aguA. The putrescine carbamoyl transferase encoded by aguB further metabolizes N-carbamoylputrescine to generate putrescine and carbamoyl phosphate. Carbamate kinase, encoded by the aguC gene, transfers a phosphate group from carbamoyl phosphate to adenosine diphosphate to generate adenosine triphosphate, CO2, and ammonia. AgDS is present in multiple oral bacteria, including S mutans, Streptococcus sobrinus, Streptococcus downeii, S rattus, Streptococcus uberis, S mitis, Streptococcus cricetus, S sanguinis, and Streptococcus salivarius, as well as Lactobacillus salivarius and Lactobacillus brevis.17 Researchers have recognized that arginine metabolism using AgDS cannot generate enough base to increase the microenvironment pH of oral biofilms; rather, its main function is to enhance bacterial acid tolerance by increasing cytoplasmic pH through ammonia and adenosine triphosphate generation.18
METABOLISM OF ARGININE IMPROVES pH HOMEOSTASIS
Although caries is a multifactorial disease involving the interactions between host, bacteria, diet, and environmental factors, the key determinant of caries is the pH decrease on the tooth surface due to bacterial fermentation–related acid production. As illustrated in the pH curves from Stephan and Miller22 in 1943, when dental plaque is exposed to fermentable carbohydrates, the pH falls rapidly, then gradually returns to starting levels. Factors such as salivary washing and alkali generation are of great importance for the pH-raising phase. Ammonia molecules produced via the ADS are protonated by the acid to produce ammonium ion (NH4+), resulting in an increase in cytoplasmic and environmental pH.23 When the pH is lower than 7.0, more than 99.4% of the ammonia molecules are protonated to ammonium.24 Neutralization of glycolytic acids by ammonia helps maintain a favorable remineralization–demineralization equilibrium on the tooth surface. Clinical observations revealed that caries-free individuals have higher concentrations of ammonia and higher resting pH values in their dental plaque compared with caries-susceptible individuals.25
Using an in vitro multispecies biofilm model established by our group, we simulated the in vivo arginine uptake and catabolism process in dental plaque. This human saliva–derived in vitro multispecies microbial community was recovered using SHI medium,26 and the cultured community contained more than 100 species of oral bacteria, thus approaching the diversity and overall metabolic functionality of the human oral microbiome.27,28 Most importantly, this in vitro system allows the accurate simulation of the pH drop and recovery in dental plaque after a glucose challenge,29 as observed in vivo. From this study, we found that treatment of in vitro biofilm with only sucrose led to a sustained pH drop from 7 to 4.5, while biofilms preincubated with arginine displayed a significant capacity to recover to a higher pH after a sucrose challenge. Our data suggests there is active uptake and metabolism of arginine within the dental biofilm, which would contribute to improved pH homeostasis, especially under acid stress.30 Consistent with our findings, Ledder and colleagues31 also demonstrated that sustained 1.5% (weight/volume) arginine treatment of an in vitro plaque microcosm significantly increased plaque pH with or without 5% sucrose supplementation.
METABOLISM OF ARGININE MODULATES BIOFILM MICROBIAL PROFILE
Findings from multiple in vitro studies have suggested that the presence of arginine may alter the biofilm species composition (Figure 1). In our in vitro biofilm model, a shift in the community structure and increased species diversity were observed in the group treated with 75 mM arginine compared to the untreated group. Furthermore, the microbial community’s capability to recover from a lowered pH after a sucrose challenge was significantly enhanced, demonstrating that the presence of arginine led to improved pH homeostasis through changes in the microbial community.30 By using S mutans, S sobrinus, and the ADS-positive (ADS+) species S sanguinis and S gordonii, Berto and colleagues32 constructed an in vitro cariogenic biofilm on enamel surfaces. The authors reported that treatment with arginine-containing sodium mono-fluorophosphate toothpaste resulted in a higher proportion of ADS+ bacterial species within the biofilm, higher culture media pH, and lower loss of enamel hardness when compared with use of the sodium mono-fluorophosphate toothpaste without arginine. In another in vitro study33 using saliva-derived biofilms, investigators reported that treatment with a combination of 2.5% arginine and 500 ppm NaF resulted in the least demineralization on the enamel surface, followed by 500 ppm NaF alone, 2.5% arginine alone, and the phosphate-buffered saline control. In addition, 2.5% arginine alone reduced the S mutans to S sanguinis ratio by inhibiting S mutans within biofilms, whereas 500 ppm NaF failed to affect the ratio because of its inhibitory effect on both S mutans and S sanguinis. This result was corroborated by other studies,30,32 supporting the ability of arginine to modulate microbial structure.
Figure 1
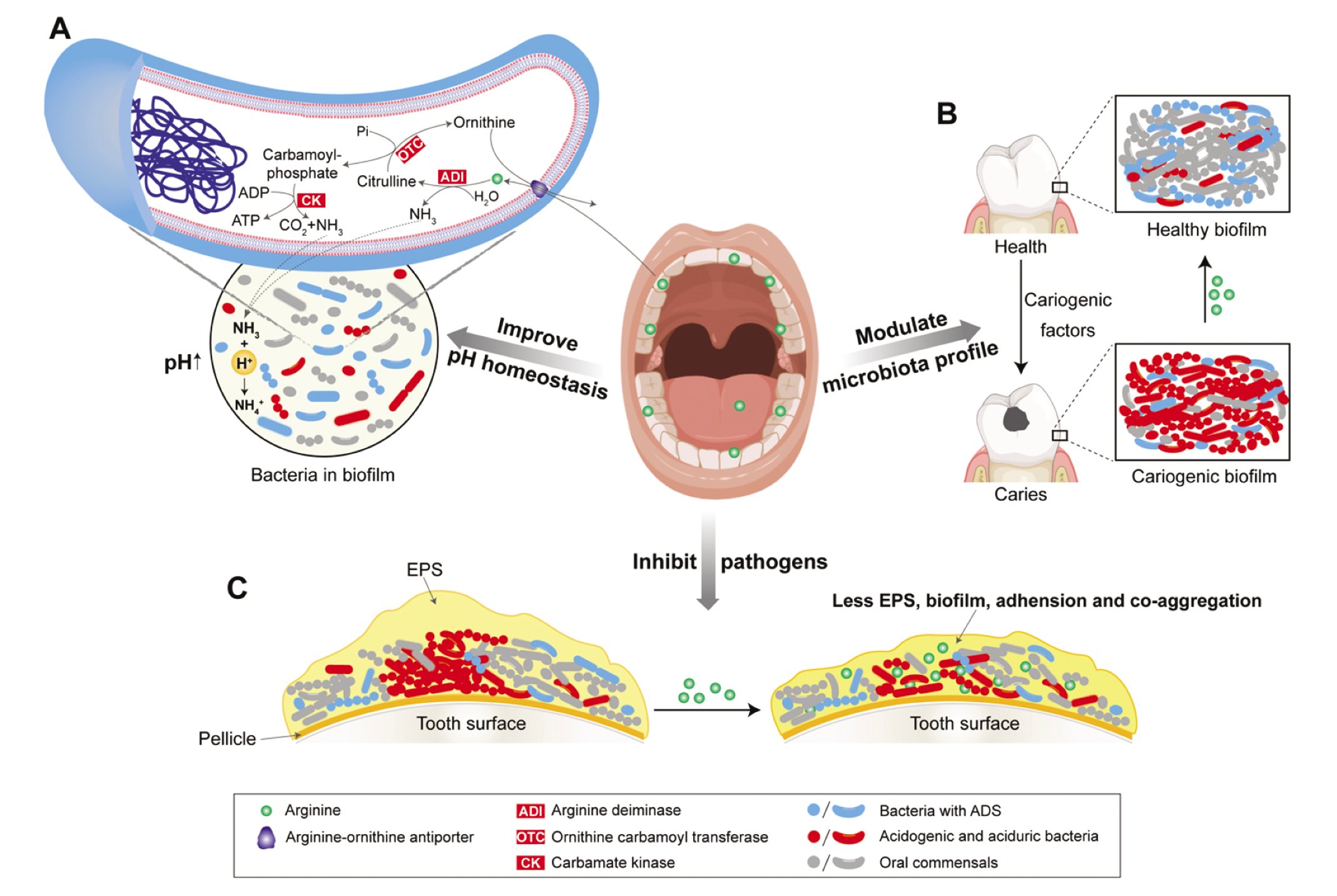
The role of arginine in caries therapeutics. A. Arginine can be transported into arginine deiminase system positive (ADS+) bacteria via arginine-ornithine antiporter and hydrolyzed by enzymes (ADI, OTC, and CK) in ADS. Metabolic products from ADS include ornithine, NH3, carbon dioxide (CO2), and adenosine triphosphate (ATP). Ammonia (NH3) picks up an H+ from the acid to produce ammonium ion (NH4+) and results in a rise in cytoplasmic and environmental pH. B. Alkali generated from arginine metabolism could modulate the microbiota profile by creating an alkaline microenvironment, facilitating the growth of oral commensals and ADS+ bacteria, while inhibiting the growth of acidogenic and acid-tolerant bacteria. C. Arginine can be taken up by dental biofilms and may directly inhibit extracellular polymeric substance (EPS) production, biofilm formation, adhesion, and co-aggregation of certain pathogens, especially acidogenic, cariogenic bacteria.
Metabolism of arginine by arginolytic bacteria contributes to an alkaline environment in dental plaque ecology, creating less favorable conditions for acidogenic and aciduric bacteria to thrive. According to the currently accepted ecological plaque hypothesis,7 bacterial metabolic acid production promotes a microenvironment favoring the growth of acidogenic and aciduric bacteria. This shift leads to excessive acid production, resulting in a more cariogenic microbiome, which drives tooth demineralization and the development of caries. Thus, alkali generated from bacterial arginine metabolism via ADS or AgDS is likely to be able to enhance the microbial ecological stability in the dental plaque by modulating the microflora profile, particularly in the presence of fermentable carbohydrates. The contribution of arginine metabolism to pH homeostasis via microbial community modulation makes it a potential candidate for effective caries prevention.
ARGININE INHIBITS BIOFILM FORMATION, ADHESION, AND CO-AGGREGATION OF CERTAIN PATHOGENS
In addition to the metabolism-related pH raising and microflora modulation capability, the presence of arginine itself may directly inhibit certain pathogens in dental biofilms, especially those recognized as cariogenic (Figure 1). Notably, arginine mainly affects the cariogenicity of S mutans by inhibiting its virulence factors rather than by directly suppressing bacterial growth.34 Studies focusing on S mutans reported that arginine could inhibit S mutans biofilm formation, adhesion, and co-aggregation, thus reducing its pathogenic potential.34-36 The mechanism underlying this biological property is the arginine-induced downregulation of genes involved in virulence, such as attachment/accumulation (gtfB and spaP), bacteriocins (nlmA, nlmB, nlmD, and cipB), and the sigma factor required for competence development (comX).34 The downregulation of these genes affects water-insoluble extracellular polymeric substance (EPS) denseness35,36 and other pathogenic abilities,34 leading to attenuated virulence. In vitro studies using multispecies biofilms with S mutans36,37 demonstrated that arginine significantly reduced the amounts of insoluble EPS and the biomass of the polymicrobial biofilms, and disrupted the dynamic microbial interactions associated with pathogenic biofilm development.
The development of dental biofilms is a well-regulated process. Fusobacterium nucleatum, considered a bridging organism, was found to play a crucial role in linking early and late colonizing bacteria during multispecies biofilm maturation.38 Kaplan and colleagues38 showed that arginine can inhibit RadD, an F nucleatum–encoded arginine-inhibitable adhesin that mediates co-aggregation between F nucleatum and early colonizing gram-positive species. Arginine was also found to inhibit co-aggregation between Porphyromonas gingivalis and mutans streptococci.39 These results suggest that arginine could interrupt cell-to-cell co-aggregation in plaque biofilms and, in doing so, reduce biofilm biomass as well as inhibit synergistic pathogenesis potentially derived from direct physical interaction among different pathogenic bacteria.
APPLICATIONS OF ARGININE IN CARIES THERAPEUTICS
Applications of arginine in caries therapeutics, particularly in caries prevention, include incorporating arginine into oral care formulations, using arginine as a prebiotic, and developing ADS+ bacteria as potential probiotics. Many arginine-based oral products have already been marketed. So far, the major goal of arginine-based products is caries prevention or treatment. Therefore, this review summarized applications and clinical outcomes of arginine-based technology in oral care, aiming to provide researchers and clinicians with the current understanding of the role and impact of arginine on caries therapeutics.
Arginine-based oral care products
The most frequently used and well-studied arginine-based products in oral care are arginine-containing dentifrices with a calcium-based abrasive system, in which arginine is either the sole effective agent or in combination with the anticaries agent fluoride. Other applications of arginine in oral care products include mouthrinses40,41 and varnishes.42 These products have been found to achieve similar anticaries effects as arginine-containing dentifrices.40-42
An in vivo study comparing 1.5% arginine, fluoride-free toothpaste with fluoride-containing toothpaste (1100 ppm fluoride as NaF) in 19 caries-free and 19 caries-active individuals twice daily for 4 weeks43 showed that arginine significantly increased ADS activity in plaque samples from the caries-active individuals. The plaque microbial profiles of caries-active participants treated with arginine displayed a shift in bacterial composition closer to the composition of caries-free participants. In another in vivo study using the same toothpaste in 83 adults, Nascimento and colleagues44 found that arginine significantly increased ADS activity and pH values of dental plaque after incubation with glucose, which was corroborated by their previous study.43 Clinically, 14% of active caries lesions became inactive after 12 weeks, although no difference was observed between arginine or fluoride treatments44. Study findings also indicated that the anticaries mechanisms of arginine and fluoride are different; while arginine metabolism promotes biofilm pH homeostasis, fluoride enhances resistance of tooth minerals to low pH, and reduces acid production in supragingival oral biofilms.44
Arginine and fluoride have anticaries therapeutic effects, albeit with different mechanisms. Researchers have proposed that the therapies complement each other, resulting in improved protection from caries. Arginine’s ability to increase microenvironmental pH and reduce biofilms’ EPS matrix may allow fluoride to be more effective in promoting tooth remineralization and allowing antimicrobials to penetrate biofilms.45 An increasing number of in vivo studies have investigated the anticaries efficacy of arginine when combined with other agents, such as fluoride and calcium, and compared its efficacy with that of fluoride only. A 2-year pivotal clinical study involving 6,000 participants showed that dentifrices containing 1.5% arginine, an insoluble calcium compound, and 1450 ppm fluoride provided significantly greater protection against caries lesion, in a low to moderate caries risk population, than dentifrices containing 1450 ppm fluoride alone.46
Studies using the same dentifrice formulations in 331 children47 and 3779 adults48 revealed that dentifrices with arginine exhibited superior efficacy in arresting and reversing active coronal caries lesions in children and active root caries lesions in adults. Meanwhile, testing of other similar dentifrices containing arginine as the effective agent, such as an arginine bicarbonate/calcium carbonate (CaviStat)–containing dentifrice, demonstrated more effective inhibition against caries initiation and progression compared with fluoride toothpaste controls.49,50 Results from meta-analyses indicated that dentifrices containing 5000 ppm fluoride or 1.5% arginine plus 1450 ppm fluoride were more effective in inactivating root caries lesions than dentifrices containing 1100 to 1450 ppm fluoride,51 suggesting that arginine could enhance the efficacy of fluoride while reducing potential side effects of high concentrations of fluoride, including fluoride toxicity and dental fluorosis.52
In in vivo anticaries dentifrice studies, the most frequently used concentration of arginine was 1.5%, which achieved similar anticaries efficacy as that in studies using 8% arginine.33,53 Additionally, in a clinical study,54 use of toothpaste containing 8% arginine, calcium carbonate, and 1450 ppm fluoride significantly reduced tooth sensitivity compared to a leading toothpaste containing 2% potassium ions. Mechanisms behind tooth hypersensitivity relief include physical sealing of dentin tubules with plugs that contain arginine, calcium carbonate, and phosphate.54 These plugs resist normal pulpal pressures and acid challenge and can effectively reduce the change in dentin fluid flow, which is the primary cause of hypersensitivity.
Arginine functions like a prebiotic
Prebiotics are defined as substrates that are selectively used by host microorganisms to confer a health benefit.55 L-arginine has been used as a dietary supplement to improve human health conditions such as cardiovascular diseases. Arginine is a precursor of nitric oxide, which plays important roles in vasodilatation, bacterial challenge and cytokine stimulation, and platelet aggregation.56 In a 1-year study,57 use of a sugarless tablet containing CaviStat (an arginine bicarbonate calcium carbonate complex) led to inhibition of caries onset and progression. Thus, arginine may also serve as a promising prebiotic in oral care.
Despite most in vivo clinical studies showing effective anticaries outcomes with no potential risks, there are 2 concerns related to using arginine in oral care products58: (1) Many studies were sponsored by companies manufacturing the tested products, which has the potential to increase the bias, although this is not uncommon in pharmaceutical trials and is an inherent and necessary part of therapeutic development; (2) Some studies raised potential concerns about arginine-related adverse effects, including discomfort, oral hygiene deterioration, tooth staining, increased periodontal disease, and oral malodor.58,59 Hence, the long-term efficacy and safety of these arginine-based clinical products require further investigation in high-quality, well-designed, non–industry-supported clinical studies.
Probiotic potential of ADS+ bacteria
A probiotic is defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host.”60 Use of probiotics in improving gastrointestinal health has a long history and is highly commercialized.60 More recently, attention has turned to the role of probiotics in oral health, particularly in caries, periodontitis, and oral candidiasis therapeutics.6 The presence of ADS varies between different species, particularly in Streptococcus and Actinomyces species, as well as between different strains of individual species.61-63 Probiotics containing ADS+ bacteria, intending to increase arginine catabolism activity within dental plaque, could be effective in preventing or even reversing the cariogenic effect of biofilms. Some studies63,64 investigated the potential of arginolytic, commensal streptococci as probiotics that could have a positive impact on the oral microbiome by increasing the local pH while antagonizing cariogenic pathogens. However, candidate strains need to be carefully selected and extensively tested before being considered for clinical applications.
CONCLUSION
Arginine is a promising anticaries therapeutic with well-documented caries prevention ability. Its anticaries properties can be attributed to the following key mechanisms: (1) arginine catabolism by arginolytic bacteria, which improves pH stability and modulates the biofilm microbiota through ammonia production; and (2) arginine-mediated inhibition of adhesion and co-aggregation and reduction of certain cariogenic bacteria in biofilms. Arginine-based oral care formulations, arginine as a prebiotic, and probiotics including ADS+ commensal bacteria are 3 potential applications that can bring arginine-based approaches into caries therapeutics. To date, arginine-based oral health products have demonstrated satisfactory in vivo efficacy in preventing and even reversing early enamel carious lesions.
Future exploration of arginine applications in caries prevention should focus on the following: (1) combining arginine therapies with existing fluoride therapies to achieve better anticaries efficacy; (2) developing arginine-based products as alternative anticaries options for individuals seeking fluoride-free products and children at risk of dental fluorosis; (3) exploring the application of arginine as a prebiotic in tandem with ADS+ bacterial strains as probiotics in the oral cavity to achieve long-term microflora ecological homeostasis through the ability of arginine to promote the growth of ADS+ bacterial species; and (4) combined application of arginine with targeted antimicrobial treatments to suppress cariogenic bacteria in a long-lasting, stable, anticaries effect because arginine can help maintain a healthy biofilm microenvironment after elimination of cariogenic bacteria. Although the potential of arginine developing into an anticaries therapeutic is clearly demonstrated by in vitro and in vivo data, more clinical studies are needed to evaluate long-term anticaries efficacy and potential adverse effects before commercializing arginine-based products.
Disclosure
Dr. Shi is a former chief science officer at C3J Therapeutics, which has licensed technologies from the University of California Regents that could be indirectly related to this research project. The authors acknowledge the efforts of Dr. Anne C.R. Tanner and Dr. Daniel Ferrer in reviewing the manuscript. Publication of this article was supported by Colgate-Palmolive Company.
References
- Bernabe E, Marcenes W, Hernandez CR, et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the Global Burden of Disease 2017 study. J Dent Res. 2020;99(4):362-373.
- Whelton HP, Spencer AJ, Do LG, Rugg-Gunn AJ. Fluoride revolution and dental caries: evolution of policies for global use. J Dent Res. 2019;98(8):837-846.
- Gonzalez-Cabezas C, Fernandez CE. Recent Advances in remineralization therapies for caries lesions. Adv Dent Res. 2018;29(1):55-59.
- Szafrański SP, Winkel A, Stiesch M. The use of bacteriophages to biocontrol oral biofilms. J Biotechnol. 2017;250:29-44.
- Baker JL, He X, Shi W. Precision reengineering of the oral microbiome for caries management. Adv Dent Res. 2019;30(2): 34-39.
- Hoare A, Marsh PD, Diaz PI. Ecological therapeutic opportunities for oral diseases. Microbiol Spectr. 2017;5(4).
- Takahashi N. Oral microbiome metabolism: from “who are they?” to “what are they doing?” J Dent Res. 2015;94(12): 1628-1637.
- Taubman MA, Nash DA. The scientific and public-health imperative for a vaccine against dental caries. Nat Rev Immunol. 2006;6(7):555-563.
- Luiking YC, Ten Have GAM, Wolfe RR, Deutz NEP. Arginine de novo and nitric oxide production in disease states. Am J Physiol Endocrinol Metab. 2012;303(10):E1177-89.
- Van Wuyckhuyse BC, Perinpanayagam HE, Bevacqua D, et al. Association of free arginine and lysine concentrations in human parotid saliva with caries experience. J Dent Res. 1995;74(2):
686-690. - Kanapka JA, Kleinberg I. Catabolism of arginine by the mixed bacteria in human salivary sediment under conditions of low and high glucose concentration. Arch Oral Biol. 1983;28(11):
1007-1015. - Wijeyeweera RL, Kleinberg I. Arginolytic and ureolytic activities of pure cultures of human oral bacteria and their effects on the pH response of salivary sediment and dental plaque in vitro. Arch Oral Biol. 1989;34(1):43-53.
- Kleinberg I KJ, Chatterjee R, Craw D, D’Angelo NK, Sandham HG. Metabolism of nitrogen by the oral mixed bacteria. In: Kleinberg I, Ellison SA, Mandel ID, eds. Saliva and Dental Caries. Information Retrieval; 1979:357-377.
- Higham SM, Edgar WM. Human dental plaque pH, and the organic acid and free amino acid profiles in plaque fluid, after sucrose rinsing. Arch Oral Biol. 1989;34(5):329-334.
- Nascimento MM, Gordan VV, Garvan CW, Browngardt CM, Burne RA. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol. 2009;24(2):
89-95. - Nascimento MM, Liu Y, Kalra R, et al. Oral arginine metabolism may decrease the risk for dental caries in children. J Dent Res. 2013;92(7):604-608.
- Liu YL, Nascimento M, Burne RA. Progress toward understanding the contribution of alkali generation in dental biofilms to inhibition of dental caries. Int J Oral Sci. 2012;4(3):135-140.
- Griswold AR, Nascimento MM, Burne RA. Distribution, regulation and role of the agmatine deiminase system in mutans streptococci. Oral Microbiol Immunol. 2009;24(1):79-82.
- Marquis RE, Bender GR, Murray DR, Wong A. Arginine deiminase system and bacterial adaptation to acid environments. Appl Environ Microbiol. 1987;53(1):198-200.
- Burne RA, Marquis RE. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett. 2000;193(1):1-6.
- Xuedong Z, ed. Dental Caries: Principles and Management. Springer; 2016.
- Stephan RM, Miller BF. A quantitative method for evaluating physical and chemical agents which modify production of acids in bacterial plaques on human teeth. J Dent Res. 1943;22(1):
45-51. - Walter A, Gutknecht J. Permeability of small nonelectrolytes through lipid bilayer membranes. J Membr Biol. 1986;90(3):
207-217. - Kunz RG. Environmental Calculations: A Multimedia Approach. Wiley; 2009.
- Margolis HC, Duckworth JH, Moreno EC. Composition of pooled resting plaque fluid from caries-free and caries-susceptible individuals. J Dent Res. 1988;67(12):1468-1475.
- Tian Y, He X, Torralba M, et al. Using DGGE profiling to develop a novel culture medium suitable for oral microbial communities. Mol Oral Microbiol. 2010;25(5):357-367.
- Edlund A, Yang Y, Hall AP, et al. An in vitro biofilm model system maintaining a highly reproducible species and metabolic diversity approaching that of the human oral microbiome. Microbiome. 2013;1(1):25.
- Edlund A, Garg N, Mohimani H, et al. Metabolic fingerprints from the human oral microbiome reveal a vast knowledge gap of secreted small peptidic molecules. mSystems. 2017;2(4):
e00058-17. - Edlund A, Yang Y, Yooseph S, et al. Meta-omics uncover temporal regulation of pathways across oral microbiome genera during in vitro sugar metabolism. ISME J. 2015;9(12):2605-2619.
- Agnello M, Cen L, Tran NC, et al. Arginine improves pH homeostasis via metabolism and microbiome modulation.
J Dent Res. 2017;96(8):924-930. - Ledder RG, Mistry H, Sreenivasan PK, Humphreys G, McBain AJ. Arginine exposure decreases acidogenesis in long-term oral biofilm microcosms. mSphere. 2017;2(4):e00295-17.
- Berto LA, Lauener A, Carvalho TS, Lussi A, Eick S. In vitro effects of arginine-containing toothpastes on cariogenic biofilms. Oral Health Prev Dent. 2019;17(4):375-383.
- Zheng X, He J, Wang L, et al. Ecological effect of arginine on oral microbiota. Sci Rep. 2017;7(1):7206.
- Chakraborty B, Burne RA. Effects of arginine on Streptococcus mutans growth, virulence gene expression, and stress tolerance. Appl Environ Microbiol. 2017;83(15):e00496-17.
- Sharma S, Lavender S, Woo J, et al. Nanoscale characterization of effect of L-arginine on Streptococcus mutans biofilm adhesion by atomic force microscopy. Microbiology. 2014;160(7):
1466-1473. - Huang X, Zhang K, Deng M, et al. Effect of arginine on the growth and biofilm formation of oral bacteria.
Arch Oral Biol. 2017;82:256-262. - He J, Hwang G, Liu Y, et al. L-arginine modifies the exopolysaccharide matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J Bacteriol. 2016;198(19):2651-2661.
- Kaplan CW, Lux R, Haake SK, Shi W. The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Mol Microbiol. 2009;71(1):35-47.
- Kamaguchi A, Baba H, Hoshi M, Inomata K. Coaggregation between Porphyromonas gingivalis and mutans streptococci. Microbiol Immunol. 1994;38(6):457-460.
- Wang XL, Cheng CY, Peng D, Wang B, Gan YH. Dental plaque pH recovery effect of arginine bicarbonate rinse in vivo. Chin J Dent Res. 2012;15(2):115-120.
- Ikeda K, Ejima D, Arakawa T, Koyama AH. Protein aggregation suppressor arginine as an effective mouth cleaning agent. Int J Biol Macromol. 2019;122:224-227.
- Shapira J, Sgan-Cohen HD, Stabholz A, et al. Clinical and microbiological effects of chlorhexidine and arginine sustained-release varnishes in the mentally retarded. Spec Care Dentist. 1994;14(4):158-163.
- Nascimento MM, Browngardt C, Xiaohui X, et al. The effect of arginine on oral biofilm communities. Mol Oral Microbiol. 2014;29(1):45-54.
- Nascimento MM, Alvarez AJ, Huang X, et al. Metabolic profile of supragingival plaque exposed to arginine and fluoride. J Dent Res. 2019;98(11):1245-1252.
- Kolderman E, Bettampadi D, Samarian D, et al. L-arginine destabilizes oral multi-species biofilm communities developed in human saliva. PloS One. 2015;10(5):e0121835.
- Kraivaphan P, Amornchat C, Triratana T, et al. Two-year caries clinical study of the efficacy of novel dentifrices containing 1.5% arginine, an insoluble calcium compound and 1,450 ppm fluoride. Caries Res. 2013;47(6):582-590.
- Srisilapanan P, Korwanich N, Yin W, et al. Comparison of the efficacy of a dentifrice containing 1.5% arginine and 1450 ppm fluoride to a dentifrice containing 1450 ppm fluoride alone in the management of early coronal caries as assessed using quantitative light-induced fluorescence. J Dent. 2013;41
(suppl 2):S29-S34. - Souza ML, Cury JA, Tenuta LM, et al. Comparing the efficacy of a dentifrice containing 1.5% arginine and 1450 ppm fluoride to a dentifrice containing 1450 ppm fluoride alone in the management of primary root caries. J Dent. 2013;41
(suppl 2):S35-S41. - Acevedo AM, Machado C, Rivera LE, Wolff M, Kleinberg I. The inhibitory effect of an arginine bicarbonate/calcium carbonate CaviStat-containing dentifrice on the development of dental caries in Venezuelan school children. J Clin Dent. 2005;16(3):
63-70. - Kleinberg I. A new saliva-based anticaries composition. Dent Today. 1999;18(2):98-103.
- Wierichs RJ, Meyer-Lueckel H. Systematic review on noninvasive treatment of root caries lesions. J Dent Res. 2015;94(2):261-271.
- Ullah R, Zafar MS, Shahani N. Potential fluoride toxicity from oral medicaments: a review. Iran J Basic Med Sci. 2017;20(8):
841-848. - Koopman JE, Hoogenkamp MA, Buijs MJ, et al. Changes in the oral ecosystem induced by the use of 8% arginine toothpaste. Arch Oral Biol. 2016;73:79-87.
- Cummins D. Dentin hypersensitivity: from diagnosis to a breakthrough therapy for everyday sensitivity relief. J Clin Dent. 2009;20(1):1-9.
- Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491-502.
- Morris SM Jr. Arginine metabolism revisited. J Nutr. 2016;146(12):2579S-2586S.
- Acevedo AM, Montero M, Rojas-Sanchez F, et al. Clinical evaluation of the ability of CaviStat in a mint confection to inhibit the development of dental caries in children. J Clin Dent. 2008;19(1):1-8.
- Li J, Huang Z, Mei L, Li G, Li H. Anti-caries effect of arginine-containing formulations in vivo: a systematic review and meta-analysis. Caries Res. 2015;49(6):606-617.
- Nascimento MM. Potential uses of arginine in dentistry. Adv Dent Res. 2018;29(1):98-103.
- Young VB. The role of the microbiome in human health and disease: an introduction for clinicians. BMJ. 2017;356:j831.
- Brailsford SR, Tregaskis RB, Leftwich HS, Beighton D. The predominant Actinomyces spp. isolated from infected dentin of active root caries lesions. J Dent Res. 1999;78(9):1525-1534.
- Huang X, Palmer SR, Ahn SJ, et al. A highly arginolytic Streptococcus species that potently antagonizes Streptococcus mutans. Appl Environ Microbiol. 2016;82(7):2187-2201.
- Huang X, Browngardt CM, Jiang M, et al. Diversity in antagonistic interactions between commensal oral streptococci and Streptococcus mutans. Caries Res. 2018;52(1-2):88-101.
- Velsko IM, Chakraborty B, Nascimento MM, Burne RA, Richards VP. Species designations belie phenotypic and genotypic heterogeneity in oral streptococci. mSystems. 2018;3(6):
e00158-18.
