Anti-Caries Mechanisms of Action of Arginine
As scientists unravel the intricacies of the oral microbial ecology, new ways in which arginine can influence biofilm ecology and health likely will emerge.
ABSTRACT
Background: The potential for arginine to impact the oral microbiome and caries was recognized in the 1970s. Over the ensuing decades much insight was gained on how arginine was metabolized by the oral microbiome. More recent clinical and laboratory-based studies revealed potent anticaries activities of arginine and began to elucidate the mechanisms by which arginine may impact caries development13-18. This paper summarizes salient findings related to metabolism of arginine by the oral microbiome in the context of dental caries.
(The articles in this JADA+ series on arginine were presented at a symposium and did not undergo peer review.)
Key Considerations: The primary mechanisms of action of arginine are almost entirely dependent on the biochemical and physiologic activities of the oral microbiome. In vitro and in vivo studies of the effects of arginine on caries and the microbiome demonstrate that arginine can be a highly effective anticaries agent on its own, or in combination with fluoride. As scientists unravel the intricacies of the oral microbial ecology, we are likely to continue to discover new ways in which arginine can influence biofilm ecology and health. Given the combination of the clinical successes with arginine to date, the growing demand for noninvasive prevention and treatment approaches for caries, and the potential that arginine can impact other diseases (periodontal diseases), respiratory infections and overall host responses by promoting the persistence of beneficial organisms in oral biofilms, research must continue apace to create a thorough understanding of what are likely many different mechanisms of action of arginine on human health.
BACKGROUND
The most common oral infectious diseases, dental caries and periodontitis, affect roughly half of the world’s population and consume tremendous resources for prevention and treatment.1 While these 2 infectious diseases have some very different characteristics, they share a common feature when viewed from the perspective of the oral microbiota: Both dental caries and periodontitis are ecologically driven diseases.2-4 The oral microbiomes associated with healthy sites in the mouth differ in microbial composition and biological activities from diseased sites. In the case of caries, health-associated sites are dominated by commensal streptococci, such as Streptococcus sanguinis and Streptococcus gordonii.5 This is also the case for a healthy periodontium, although there are some important distinctions between supra- and subgingival microbiomes.6-7 Initiation and progression of a carious lesion is driven almost entirely by fermentation to acidic end products by the oral microbiota of carbohydrates ingested repeatedly by the host. The repeated acidification of tooth biofilms dissolves tooth mineral but also selects for a microbial population that is better able to grow and to metabolize sugars at lower pH values than health-associated microbes—a characteristic known as aciduricity. As carious lesions worsen, the proportions of highly aciduric microbes—S mutans, Lactobacillus spp., Scardovia spp., and others—continue to increase at the expense of many health-associated species.5 Likewise, in periodontal diseases, the progression of a lesion is accompanied by a decrease in the proportions of many gram-positive, sugar-utilizing bacteria and their replacement with strongly proteolytic gram-negative bacteria that can undermine host defenses and elicit tissue damage with a wide variety of biological molecules.6,7
Research conducted by basic, translational, and clinical scientists over the years has focused primarily on the organisms that show the strongest association with disease, referred to as pathogens, opportunistic pathogens, or pathobionts. Collectively, these studies have yielded a much better understanding of how the organisms associated with active disease—heretofore referred to simply as “pathogens”—establish, persist, and initiate or worsen disease. In contrast, the amount of research conducted on health-associated organisms, even those that are the most abundant species in oral biofilms, is only a small fraction of that focused on pathogens. Moreover, a substantial portion of the studies on the health-associated oral organisms have been focused on the role of these microbes in extraoral infectious diseases, eg, viridans streptococci in endocarditis. This asymmetry in knowledge was created not because there was a lack of recognition that the health-associated organisms might be doing things that are beneficial; for example, see reference 8. Rather, it was primarily attributable to logically organized priorities of clinicians, scientists, and funding agencies—why would you not most vigorously study the organisms that are present when there is evidence of disease? Fortunately, as DNA sequencing and other technologies have rapidly advanced, the foundation and rationale for devoting more effort to understanding the probiotic effects of potentially beneficial bacteria—ie, how health-associated bacteria may promote a healthy mouth and body—has been greatly strengthened. And, in concert with mechanistic studies, it is becoming clear that many of the health-associated commensal bacteria actively engage in modifying oral biofilm environments to make them more compatible with maintaining the integrity of the adjacent tissues and less favorable for the establishment or outgrowth of pathogenic organisms. While the statements in this paragraph apply to both caries and periodontal diseases, the remainder of this treatise will focus on dental caries.
Present therapeutic and treatment strategies for caries largely ignore the critical role that intermicrobial interactions and microbial ecology play in the development and progression of the disease. Vaccination has fallen by the wayside, while biofilm removal and excavation of damaged tissue have remained the standard of care, although major strides have been made in the nonsurgical management of caries. Recently, though, basic and clinical research has highlighted that incorporation of arginine into oral health care formulations is a promising novel approach to caries prevention and management.
The potential for arginine to impact the oral microbiome and caries was recognized in the 1970s. In the early 1980s, Kleinberg and colleagues demonstrated that arginine, as opposed to the other 19 biologically common amino acids, could serve particularly well as a substrate for mixtures of the oral microbiota from human subjects to generate ammonia (pKa = 9.2)9-12, as well as carbon dioxide. Specifically, when saliva was collected from volunteers and the bacteria were exposed to arginine or small arginine-containing peptides, a rapid rise in the pH was evident. Concomitant metabolism of arginine and sugar blunted acidification of the mixtures, whereas mixtures provided only with glucose rapidly reduced the pH. Over the ensuing decades, much insight was gained into how arginine was metabolized by the oral microbiome. More recent clinical and laboratory-based studies revealed potent anti-caries activities of arginine and began to elucidate the mechanisms by which arginine may impact caries development.13-18 Here, we summarize salient findings related to metabolism of arginine by the oral microbiome in the context of dental caries.
Mechanism of action of arginine against caries
If there is only one take-away from this article (hopefully not), it should be that the primary mechanisms of action of arginine are almost entirely dependent on the biochemical and physiologic activities of the oral microbiome. That is not to say that arginine may not possess certain physicochemical properties that could allow the amino acid itself to interact with other factors on its own to influence disease development. However, from what is known at this time, such effects are probably minor compared to the impact that the bacterial metabolism of arginine has on caries formation.
Figure 1
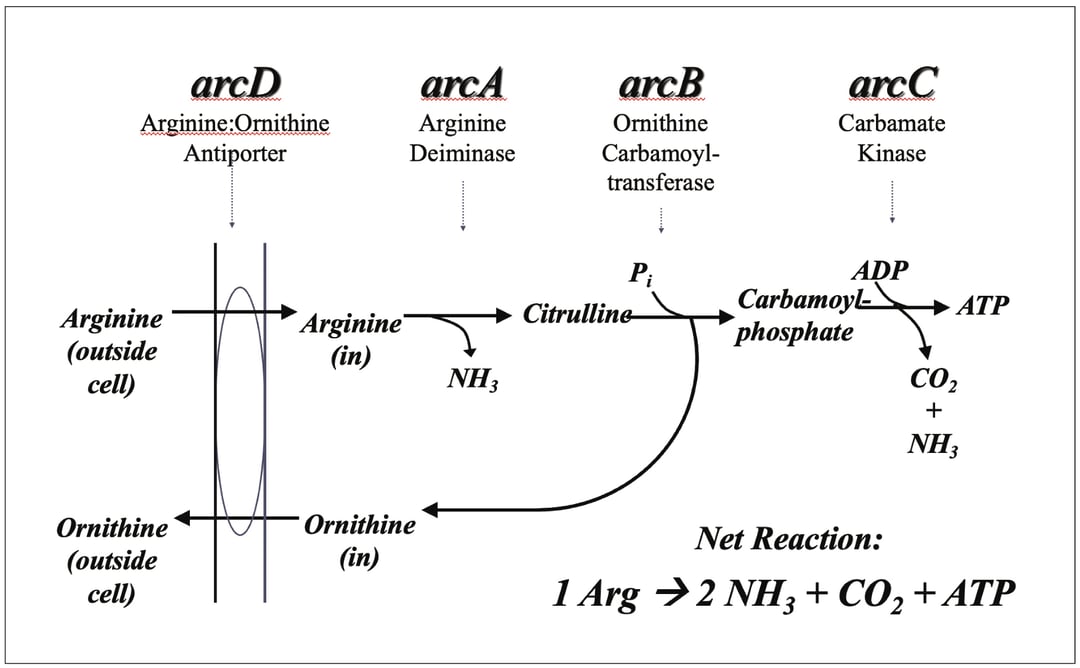
The Arginine Deiminase Pathway. The core arginine deiminase system is a 3-enzyme pathway, encoded by the arcABC genes, that converts arginine to ornithine plus 2 molecules of NH3 and one of CO2. The final step, catalyzed by carbamate kinase, uses ADP to produce ATP. The ornithine created can be exchanged for arginine outside the cell by the ArcD arginine:ornithine antiporter with little bioenergetic cost, compared, for example, to transporters that internalize compounds at the expense of ATP. Many ADS-positive bacteria also have genes for cleaving arginine from peptides and for regulating the genes in response to arginine and oxygen.
When arginine is presented to oral biofilms, the primary route of metabolism is via a 3-enzyme pathway called the arginine deiminase system (ADS), which is not the same as amino acid deamination that is carried out by a broad spectrum of organisms.8 Arginine is transported into the cell by ADS-positive bacteria, where it is acted on by the enzyme arginine deiminase (ArcA) to yield 1 molecule of ammonia and 1 of citrulline (Fig. 1). The citrulline is acted on by a catabolic ornithine transcarbamylase to produce carbamyl phosphate and ornithine. While ornithine is usually excreted from the cells, the carbamyl phosphate is cleaved in the presence of ADP to yield 1 molecule each of CO2 and ammonia, along with generation of ATP. Use of arginine by the ADS has multiple consequences that are directly related to its anticariogenic potential. First, the ammonia that is released plays two major roles. First, ammonia (uncharged NH3) can rapidly diffuse through the cell membrane and into the surrounding biofilm matrix (Fig. 2). NH3 rapidly equilibrates to ammonium ion (NH4+) by complexing with a proton (H+), which raises the environmental pH. The increased pH does two important things. First, it reduces the tooth demineralizing potential of the biofilms and enhances the remineralization potential. Secondly, the elevated pH creates a local environment that is more favorable for the less-aciduric commensals and less favorable for aciduric caries pathogens, which use their acid tolerance to outcompete health-associated bacteria at low pH. Furthermore, the ammonia released inside ADS-positive bacteria confers a bioenergetic advantage to these microbes. In particular, bacterial cells actively strive to maintain an internal (cytoplasmic) pH that is higher than the surroundings when confronted with acidic environments. A major strategy used by the streptococci is to spend ATP to pump protons (H+) out of the cell to maintain the ∆pH component of the proton motive force (pHin > pHout). Each molecule of ammonia that consumes a proton in the cell saves the cell from having to move that proton out by using ATP; the ATP can be redirected to biosynthetic processes and growth. And, of course, the final step in the ADS pathway yields ATP, which offers the organism a direct bioenergetic benefit (Fig. 2). Notably, caries pathogens routinely lack the ADS. For example, there are hundreds of strains of S mutans that have been examined biochemically or at the genome sequence, and none have the ADS genes or enzymes. Importantly, the pH and bioenergetic benefits of arginine metabolism should ultimately translate into favorable modification of oral biofilm ecology. Real-world evidence that this is the case is accumulating. By way of example, site-specific dental plaque from health subjects harbors significantly more AD activity and can generate substantially more ammonia from arginine than biofilms from carious tissues, and the more advanced the lesion, the lower the ADS activity.13 Moreover, when the microbiomes of caries-active subjects are compared with those of healthy subjects—using a technique called principal component analysis—they are fundamentally different. However, when those caries-active subjects utilize an arginine-containing (fluoride-free) dentifrice for as little as 2 weeks, a shift in the composition of the microbiome away from caries-active characteristics toward a health-associated state is observed.15
Figure 2
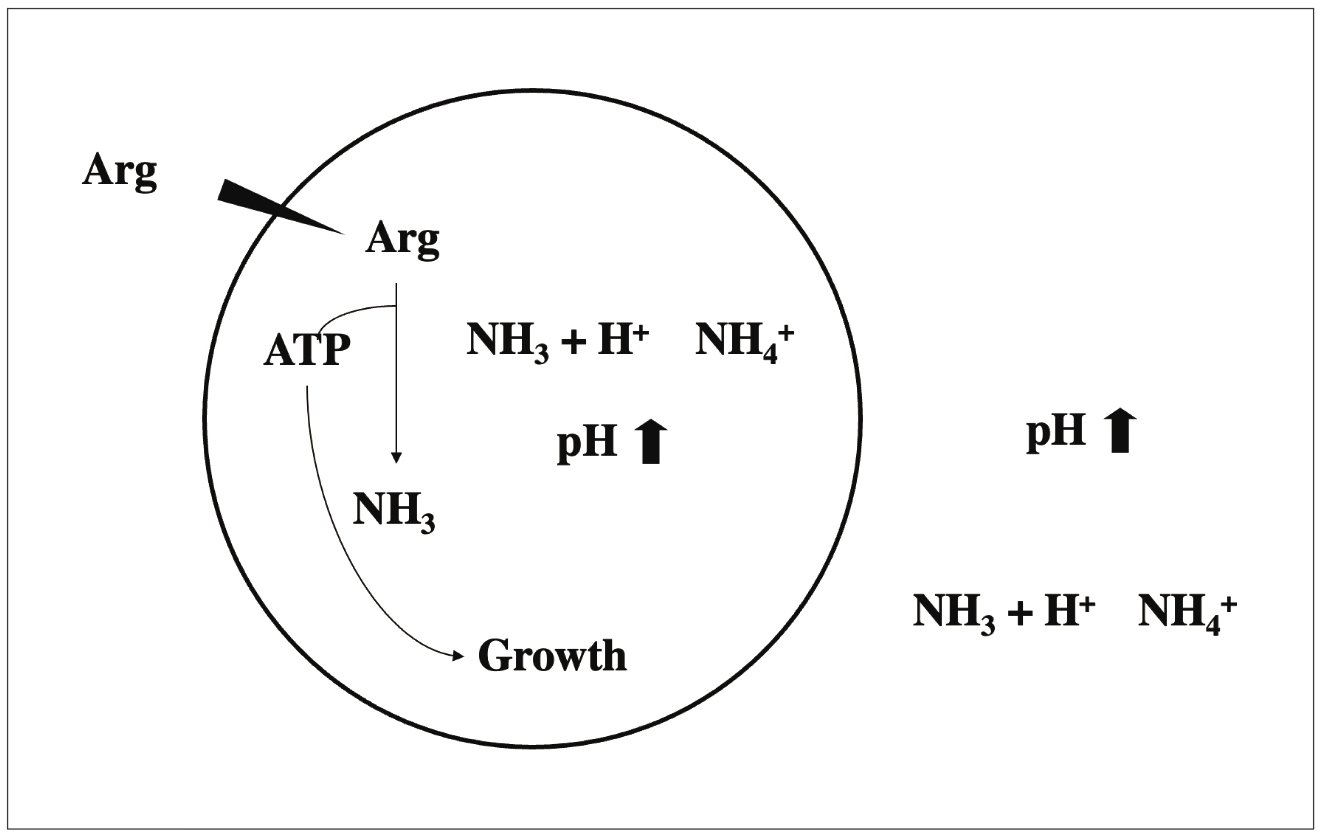
Bioenergetics of Arginine Metabolism by the ADS. Many bacteria, oral streptococci in particular, will use ATP to move protons out of the cell to maintain an internal pH that is higher than the surroundings. The ammonia released by arginine inside the cell can raise the internal (cytoplasmic) pH of the cells, saving the cells tremendous amounts of ATP to maintain ∆pH (pHin > pHout). NH3 can also diffuse rapidly through the cell membrane—it is uncharged—and raise the environmental pH. In addition, the ATP generated by arginine catabolism by the ADS can be used for growth and persistence. Thus, the ADS provides beneficial commensals with a substantial advantage when competing against caries pathogens.
Other important mechanisms of action of arginine in prevention of caries
Clearly, providing selective bioenergetic advantages to health-associated, and often overtly beneficial, members of the oral microbiota and creating a tooth biofilm environment that is less acidic are major contributions of arginine metabolism to the promotion of oral health and the inhibition of dental caries. As we delve deeper into the complexities of the interactions between health-associated and pathogenic members of the tooth microbiota, though, we are learning that the “biofilm battles”19 that influence the balance between health and disease are far more complex than previously appreciated.
First, though, it is important to highlight a key characteristic of the most abundant members of the oral microbiome: Individual isolates differ dramatically from one another, both in gene content and in phenotypic behaviors. This was first investigated in a comprehensive and systematic manner when the genomes of more than 60 isolates of S mutans from around the globe were sequenced and the data were subjected to phylogenomic analyses.20 Much was learned and continues to be learned from this endeavor, but the salient points here are that while most isolates of S mutans carry about 2000 genes, only about 1400 of those genes are present in every isolate—and if all the genes that are carried by the species S mutans are added up, the number likely exceeds 4000 at this point in time. So, every isolate of S mutans has the potential to carry about 600 genes that are not evenly distributed in the population. As importantly, phenotypic behaviors that are thought to be critical to virulence—including essential cariogenic properties like aciduricity, acidogenesis, exopolysaccharide production, and oxidative stress tolerance—differ widely between strains with different gene content.21 This genomic and phenotypic heterogeneity makes it difficult to target S mutans specifically with novel therapeutics unless they are directed at traits that are essential to initiating or worsening caries and are conserved and essential across all isolates.
Relevant to this discussion is the more recent observation that the most abundant commensal streptococci, including those that display overtly beneficial properties (eg, ADS-positive and/or inhibiting the growth of S mutans), show a similar, if not greater, degree of genomic and phenotypic heterogeneity. In the case of the commensals and S mutans, this genomic diversity has been attributed to promiscuous lateral gene transfer, as these organisms can actively take up and incorporate DNA from their surroundings into their genetic repertoire.22 The degree to which genomic heterogeneity impacts our ability to understand the output from studies of the oral microbiome is illustrated by the fact that for some oral streptococci, it is not even possible to assign them reliably to a particular species, even after the entire genome sequence is known22, which is attributable to the extensive gene exchange and genomic rearrangements that have occurred in these organisms. And, as for S mutans, the genomic heterogeneity manifests in tremendous phenotypic heterogeneity. Unfortunately, what we know now is that species that have been associated with oral health can vary widely in their capacity to express beneficial traits, such as producing alkali from arginine, creating hydrogen peroxide (H2O2) that can kill many pathogens, and interfering with the growth of S mutans in other ways. Importantly, this explains, to a degree, the difficulties that have been encountered when investigators attempt to make definitive interpretations about differences in the composition of health- and disease-associated microbiomes. For example, an individual with caries may have high levels of commensal streptococci that are typically associated with dental health, but the strain(s) with which they are colonized may lack robust probiotic properties or may even be overtly cariogenic. These challenges are now being addressed by studying large panels of isolates to understand how the presence and expression levels of particular genes can influence beneficial or pathogenic potential. Fascinating data is emerging about the internecine warfare in oral biofilms, but it turns out that arginine can influence individual bacteria and interbacterial competition in intriguing and apparently very significant ways beyond the primary MOAs described above.
Novel MOA of arginine
Direct effects on S mutans
Work from the laboratories of Rickard in Michigan and Koo in Pennsylvania explored how arginine may impact the physical characteristics of biofilms formed by S mutans using single- or mixed-species in vitro biofilm models.23,24 The takeaway from these elegant studies is that arginine itself may affect S mutans in ways that destabilize the biofilms that are formed in the presence of sucrose. Measurements of the forces that hold biofilms together and sophisticated microscopic dissection of the architecture of the biofilms formed in the presence and absence of arginine reveal significant spatiotemporal changes. A growing body of literature supports that such changes may affect the cariogenic potential of oral biofilms and that such changes would translate to diminished caries in humans, although most of the evidence is indirect. One important observation, though, is that arginine disrupted the formation of highly acidic microenvironments within the biofilms, which are key to damaging the tooth. More recently, Koo and Whiteley collaborated to image cariogenic biofilms from humans and then modeled those biofilms in vitro to provide intriguing evidence that directly supports that the way in which S mutans organizes with commensal streptococci and other bacteria may influence local acidification and thus directly affect the degree to which enamel is damaged.25 If, as it appears, arginine can disrupt these interactions, such impacts could represent a novel MOA for arginine against caries.
Concurrently, our laboratory used a somewhat different approach to explore the impact of arginine on S mutans. Using various in vitro models and high-throughput quantitative sequencing (RNA sequencing) of mRNA from S mutans cultured with or without 1.5% arginine, it was shown that arginine—though it cannot be catabolized via the ADS—decreases acid and oxidative stress tolerance, affects expression of the enzymes required for exopolysaccharide production, and changes the overall transcriptome of the organism in ways that may reduce its potential to colonize, persist, and cause caries.26 The mechanisms by which this occurs are not fully understood, as is the case for the above-refenced biofilm studies, but arginine appears to have the potential to diminish the cariogenic potential of S mutans absent the presence of ADS-positive commensals by inducing significant alterations in the expression of a large panel of genes that contribute to the virulence of S mutans.
Impacts on interbacterial competition
Antagonism of one microorganism against others that are competing to persist in the same habitat is nearly universal, whether it is in soil, in water, on shower curtains, or in the human mouth. So is cooperation between microbes. In terms of arginine’s impact on the ability of beneficial oral streptococci to interfere with growth and biological processes of S mutans, a fascinating story is developing. As noted above, there is a growing consensus that H2O2 production by commensal streptococci is an important ecological determinant, although the impacts may vary depending on the site in the mouth. In terms of caries, many commensal streptococci produce relatively high (millimolar) levels of H2O2. Whereas the commensals can tolerate such high levels of this compound, the growth and fitness of S mutans is compromised by much lower levels of H2O2. Thus, it has been posited that H2O2 production is a primary mechanism used by commensals in vivo in humans to discourage the establishment and reduce the cariogenicity of S mutans. As shown in Figure 3, H2O2 by a beneficial commensal is actually stimulated by the presence of arginine. Moreover, the increased production of H2O2 manifests in greater inhibition of S mutans by the commensal, and the enhancement in inhibition is dependent on H2O2 as addition of the enzyme catalase, which rapidly breaks down H2O2, blocks the inhibition. One could say, then, that an additional MOA of arginine may be its ability to potentiate the antagonistic effects of commensal streptococci.
More sophisticated weapons systems
We now know that many bacteria can communicate using diffusible small molecules. In gram-positive bacteria such as S mutans, these signals are usually small peptides ranging in size from as few as 7 amino acids to as large as 20 to 30 amino acids. One set of genes that is regulated by peptide signaling in S mutans is the production of bacteriocins, which are small antimicrobial peptides. Most strains of S mutans that have been examined have the capacity to produce a wide spectrum of antimicrobial molecules, often as many as 5 or 7 different compounds, that can kill or otherwise inhibit related species. For example, the widely studied S mutans strain UA159 makes at least 5 different antimicrobial compounds. Some of these antimicrobial peptides are under the control of a peptide signaling system consisting of an 18-amino acid peptide called competence-stimulating peptide (CSP) and a specific signal transduction system that detects CSP and activates the genes for the antimicrobial peptide repertoire. Interestingly, commensal oral streptococci, such as S gordonii and the highly antagonistic S sp. A12, produce a protease (Sgc) that degrades CSP and blocks S mutans’ ability to make certain factors that can kill commensals. S sp. A12, but not S gordonii or most other commensals, can also degrade a second critical peptide signal (comX-inducing peptide) and signal relay pathway (ComR) that are highly conserved in every S mutans isolate studied thus far.27 Notably, arginine can exacerbate effects of signaling and diminish the ability of S mutans to sense and respond to its own peptide signals.
Figure 3
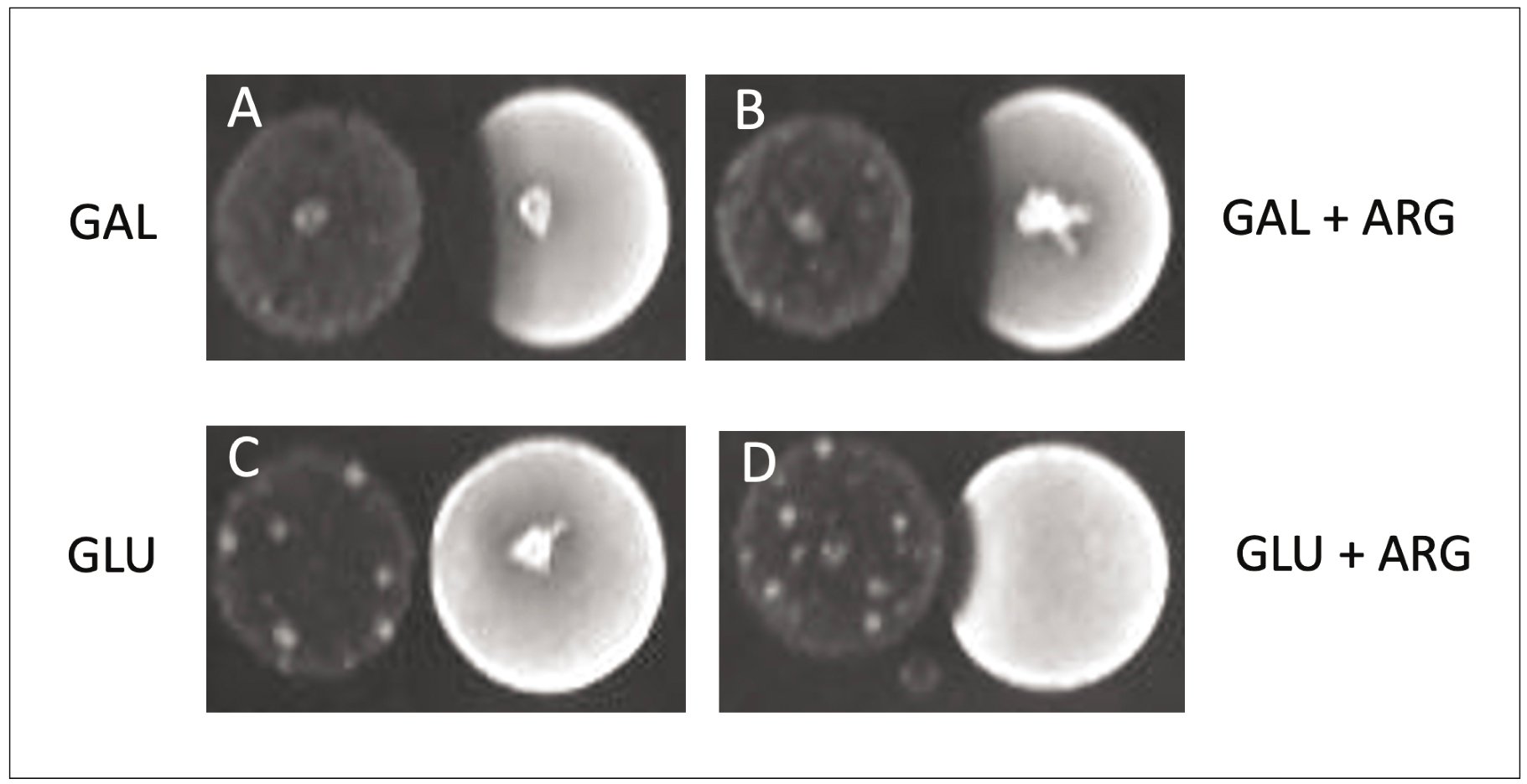
Antagonism of S mutans by a Clinical Isolate of S mitis. Cells were cultured in broth, and a small aliquot of each culture was spotted onto rich medium agar plates containing galactose (A), galactose and 1.5 % arginine (B), glucose (C), or glucose and 1.5% arginine (D). In each panel, S mitis is on the left and S mutans is on the right. Without inhibition by S mutans, the bacterial colony would be a circle. Inhibition is evident, as there is a lack of growth of S mutans where it is closest to the S mitis colony.
Summary and Future Directions
Clinicians, researchers, and lay persons have been observing dental plaque at a macroscopic and microscopic level for centuries. The use of mechanical and chemical strategies for the wholesale removal of oral biofilms has been a common practice for millennia. In the 1940s, the impact of fluoride on caries came to light, and fluoride’s ability to strengthen enamel and to promote remineralization of damaged tooth tissue have made it the gold standard for prevention of caries, coupled with regular biofilm removal. Contemporary research on oral infectious diseases now embraces, and has provided additional support for, the ecological plaque hypothesis put forward by Marsh and others.2 Slower to emerge have been technologies that can complement the action of fluoride by acting on oral biofilm ecology in ways that promote the stability of a health-associated flora—a flora with probiotic capabilities—and discourage the establishment, or reduce the cariogenicity, of aciduric pathogens. As described above, these advances have been hampered by an incomplete appreciation of the complexities of oral microbial ecology. In vitro and in vivo studies of the effects of arginine on caries and the microbiome demonstrate that arginine can be a highly effective anti-caries agent on its own or in combination with fluoride. Like many agents, for example, aspirin, recognition of the benefits has outpaced the development of a full understanding of all of the ways that the agents function. As microbiologists, biochemists, clinicians, and bioinformaticians unravel the intricacies of oral microbial ecology, we are likely to continue to discover new ways in which arginine can influence biofilm ecology and health. Given the combination of the clinical successes with arginine to date, the growing demand for noninvasive prevention and treatment approaches for caries, and the potential that arginine can impact other diseases (periodontal diseases), respiratory infections, and overall host responses by promoting the persistence of beneficial organisms in oral biofilms, research must continue apace to create a thorough understanding of what are likely many different mechanisms of action of arginine on human health.
Acknowledgments
This work was funded in part by NIH/NIDCR R01 DE25832, T90 DE21990, F30 DE28184, and a research grant from the Colgate-Palmolive Company. The author thanks Brinta Chakraborty, Kyulim Lee, and John Chase for their work on oral commensal streptococci and examination of the impacts of arginine on S mutans and Ann Griswold for assistance with Figure 2.
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018(10100);392:1789-1858.
- Marsh PD, Zaura E. Dental biofilm: ecological interactions in health and disease. J Clin Periodontol. 2017;44(Suppl 18):S12-S22.
- Marsh PD. In sickness and in health-what does the oral microbiome mean to us? An ecological perspective. Adv Dent Res. 2018;29(1):60-65.
- Burne RA, Zeng L, Ahn SJ, et al. Progress dissecting the oral microbiome in caries and health. Adv Dent Res. 2012;24(2):77-80.
- Becker MR, Paster BJ, Leys EJ, et al. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40(3):1001-1009.
- Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192(19):5002-5017.
- Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80-87.
- Burne RA, Marquis RE. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett. 2000;193(1):1-6.
- Kanapka JA, Kleinberg I. Catabolism of arginine by the mixed bacteria in human salivary sediment under conditions of low and high glucose concentration. Arch Oral Biol. 1983;28(11):1007-1015.
- Singer DL, Chatterjee R, Denepitiya L, Kleinberg I. A comparison of the acid-base metabolisms of pooled human dental plaque and salivary sediment. Arch Oral Biol. 1983;28(1):29-35.
- Wijeyeweera RL, Kleinberg I. Acid-base pH curves in vitro with mixtures of pure cultures of human oral microorganisms. Arch Oral Biol. 1989;34(1):55-64.
- Wijeyeweera RL, Kleinberg I. Arginolytic and ureolytic activities of pure cultures of human oral bacteria and their effects on the pH response of salivary sediment and dental plaque in vitro. Arch Oral Biol. 1989;34(1):43-53.
- Nascimento MM, Gordan VV, Garvan CW, Browngardt CM, Burne RA. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol. 2009;24(2):89-95.
- Nascimento MM, Liu Y, Kalra R, et al. Oral arginine metabolism may decrease the risk for dental caries in children. J Dent Res. 2013;92(7):604-608.
- Nascimento MM, Browngardt C, Xiaohui X, Klepac-Ceraj V, Paster BJ, Burne RA. The effect of arginine on oral biofilm communities. Mol Oral Microbiol. 2014;29(1):45-54.
- Nascimento MM, Alvarez AJ, Huang X, et al. Arginine metabolism in supragingival oral biofilms as a potential predictor of caries risk. JDR Clin Trans Res. 2019;4(3):262-270.
- O’Connell LM, Santos R, Springer G, Burne RA, Nascimento MM, Richards VP. Site-specific profiling of the dental mycobiome reveals strong taxonomic shifts during progression of early-childhood caries. Appl Environ Microbiol. 2020;86(7):e02825-19.
- Richards VP, Alvarez AJ, Luce AR, et al. Microbiomes of site-specific dental plaques from children with different caries status. Infect Immun. 2017;85(8):e00106-17.
- Chakraborty B, Lee K, Burne RA. Biofilm battles: beneficial commensals versus S mutans. California Dent J. 2017;45(10):547-556.
- Cornejo OE, Lefébure T, Bitar PDP, et al. Evolutionary and population genomics of the cavity causing bacteria S mutans. Mol Biol Evol. 2013;30(4):881-893.
- Palmer SR, Miller JH, Abranches J, et al. Phenotypic heterogeneity of genomically-diverse isolates of S mutans. PLoS One. 2013;8(4):e61358.
- Velsko IM, Chakraborty B, Nascimento MM, Burne RA, Richards VP. Species designations belie phenotypic and genotypic heterogeneity in oral streptococci. mSystems. 2018;3(6):e00158-18.
- He J, Hwang G, Liu Y, et al. L-arginine modifies the exopolysaccharide matrix and thwarts S mutans outgrowth within mixed-species oral biofilms. J Bacteriol. 2016;198(19):2651-2661.
- Kolderman E, Bettampadi D, Samarian D, et al. L-arginine destabilizes oral multi-species biofilm communities developed in human saliva. PLoS One. 2015;10(5):e0121835.
- Kim D, Barraza JP, Arthur RA. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc Natl Acad Sci U S A. 2020;117(22):12375-12386.
- Chakraborty B, Burne RA. Effects of arginine on S mutans growth, virulence gene expression, and stress tolerance. Appl Environ Microbiol. 2017;83(15):e00496-17.
- Huang X, Palmer SR, Ahn SJ, et al. A highly arginolytic S species that potently antagonizes Streptococcus mutans. Appl Environ Microbiol. 2016;82(7):2187-2201.
